years of active operations in the region as of August 31, 2023
approvals from the US Food & Drug Administration as of August 31, 2023
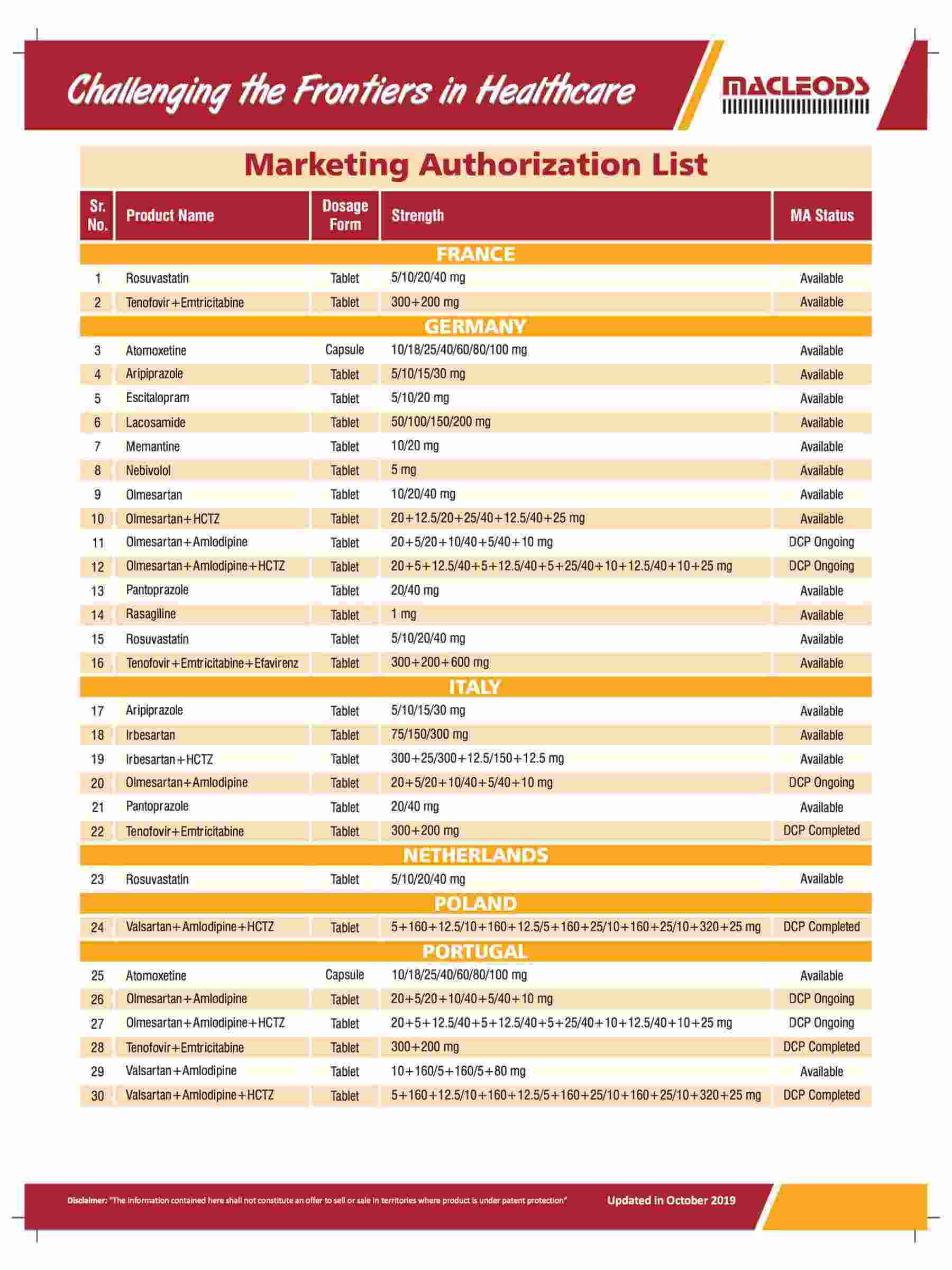
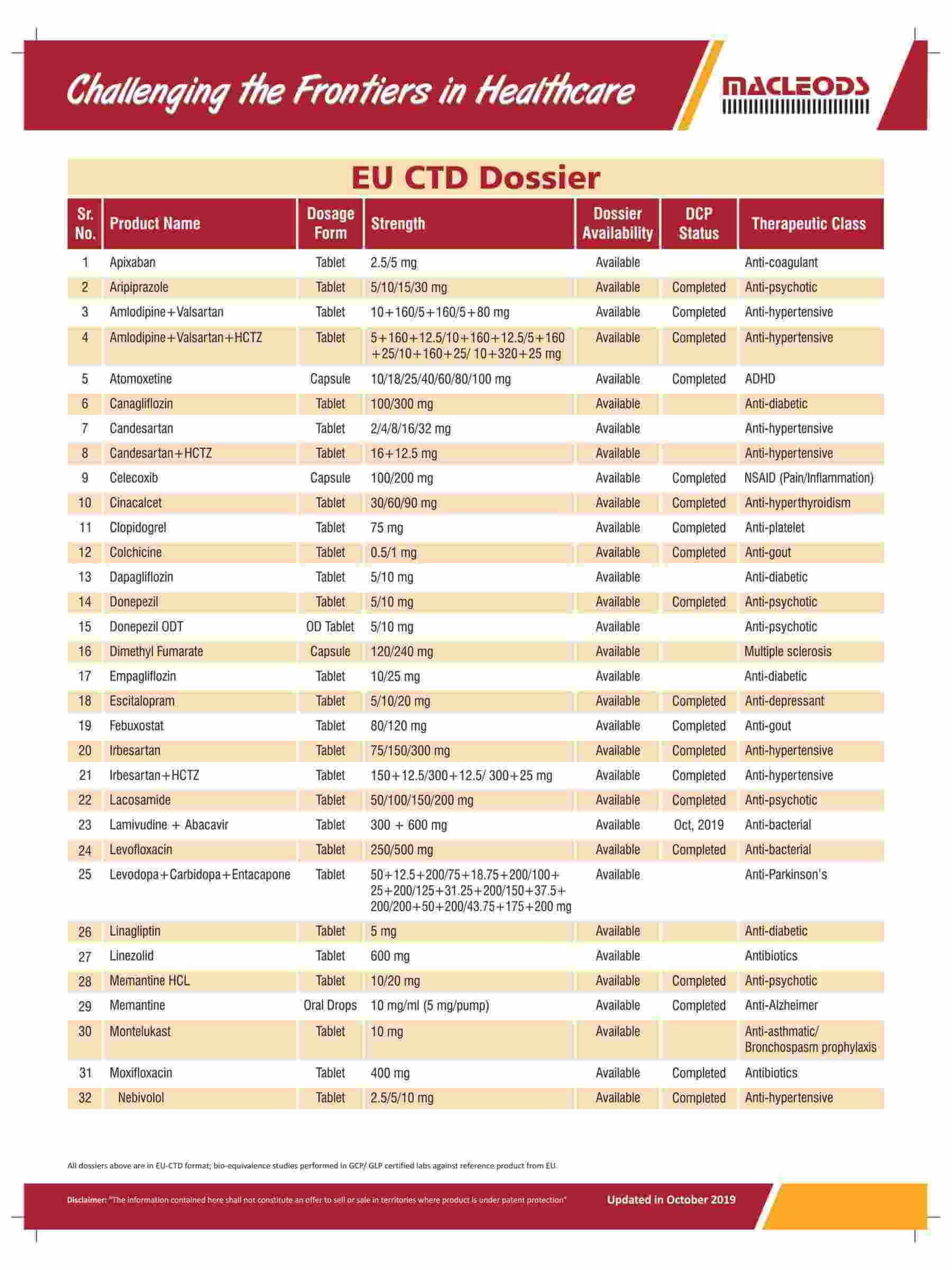
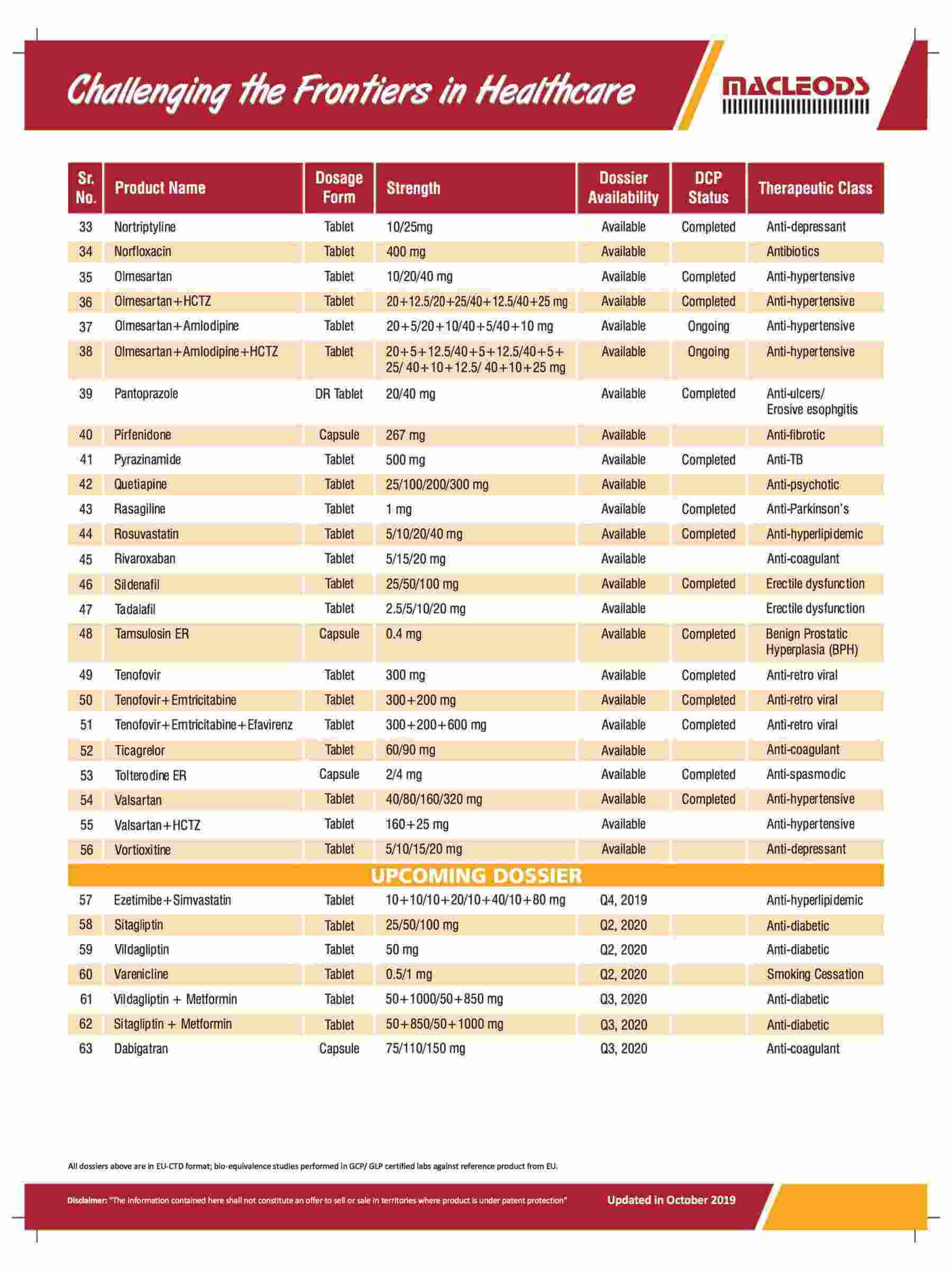
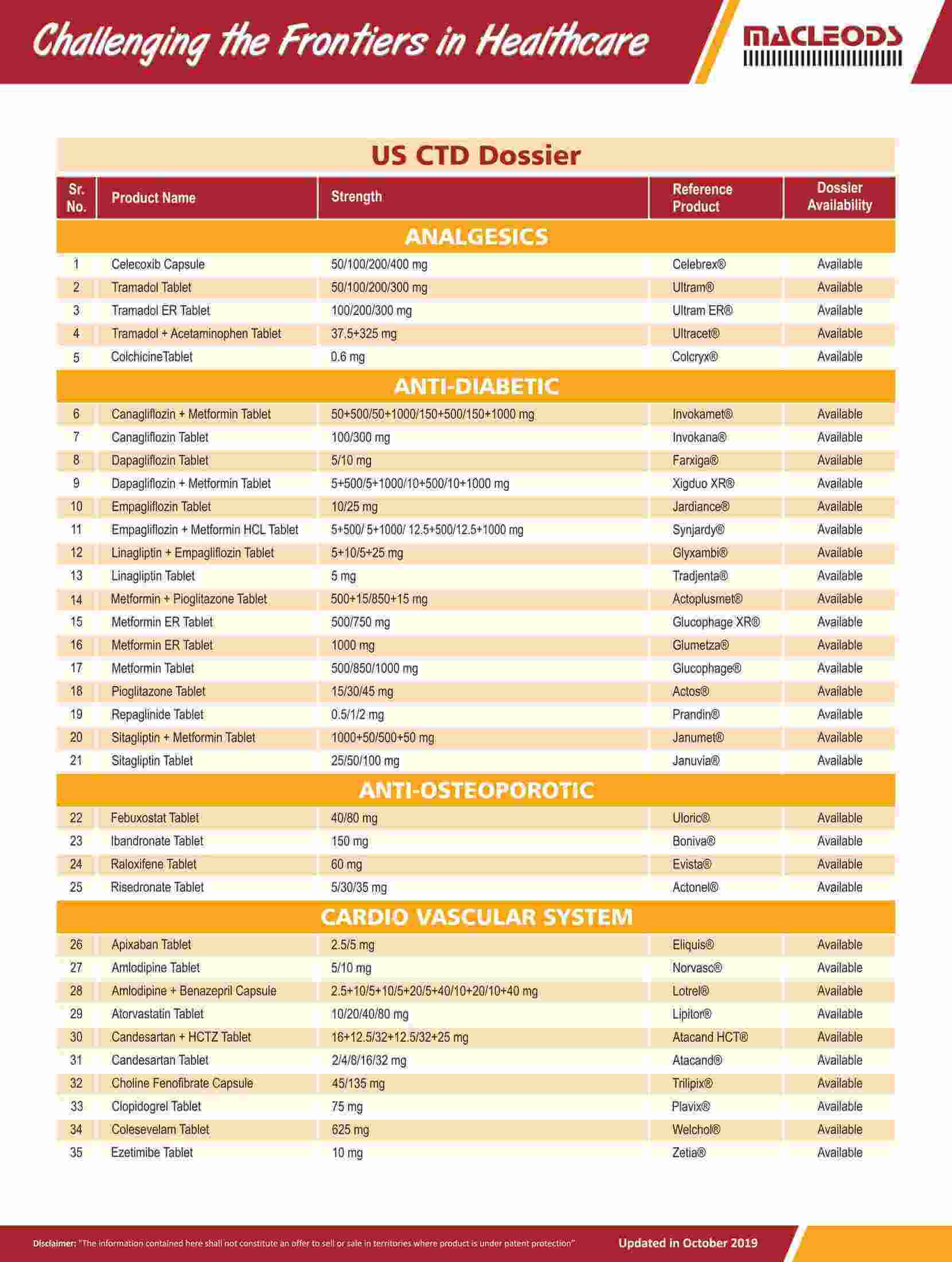
About
The formation of Macleods Pharma USA, Inc. in 2011, marked the commencement of our pharmaceutical business operations in the United States.
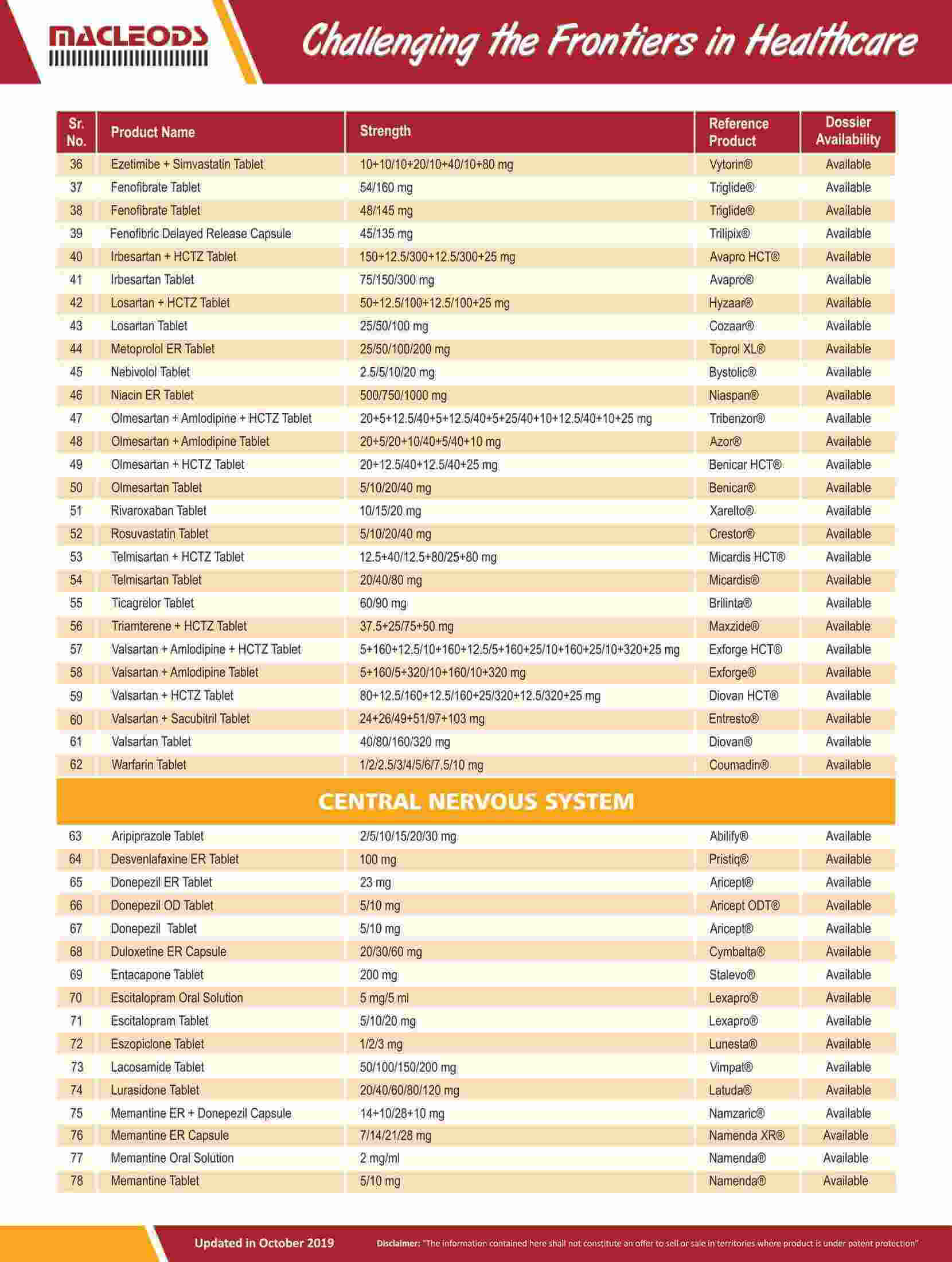
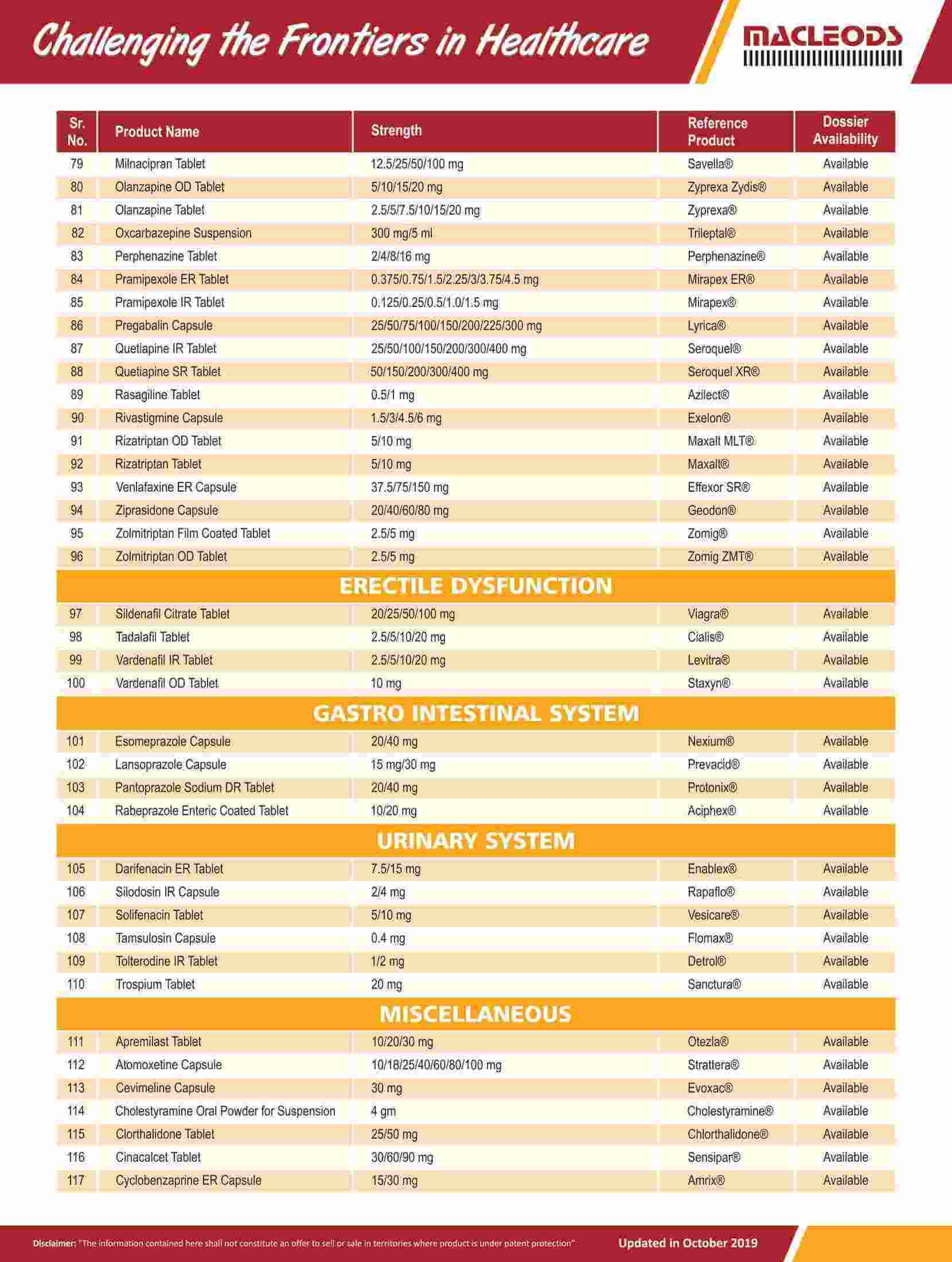
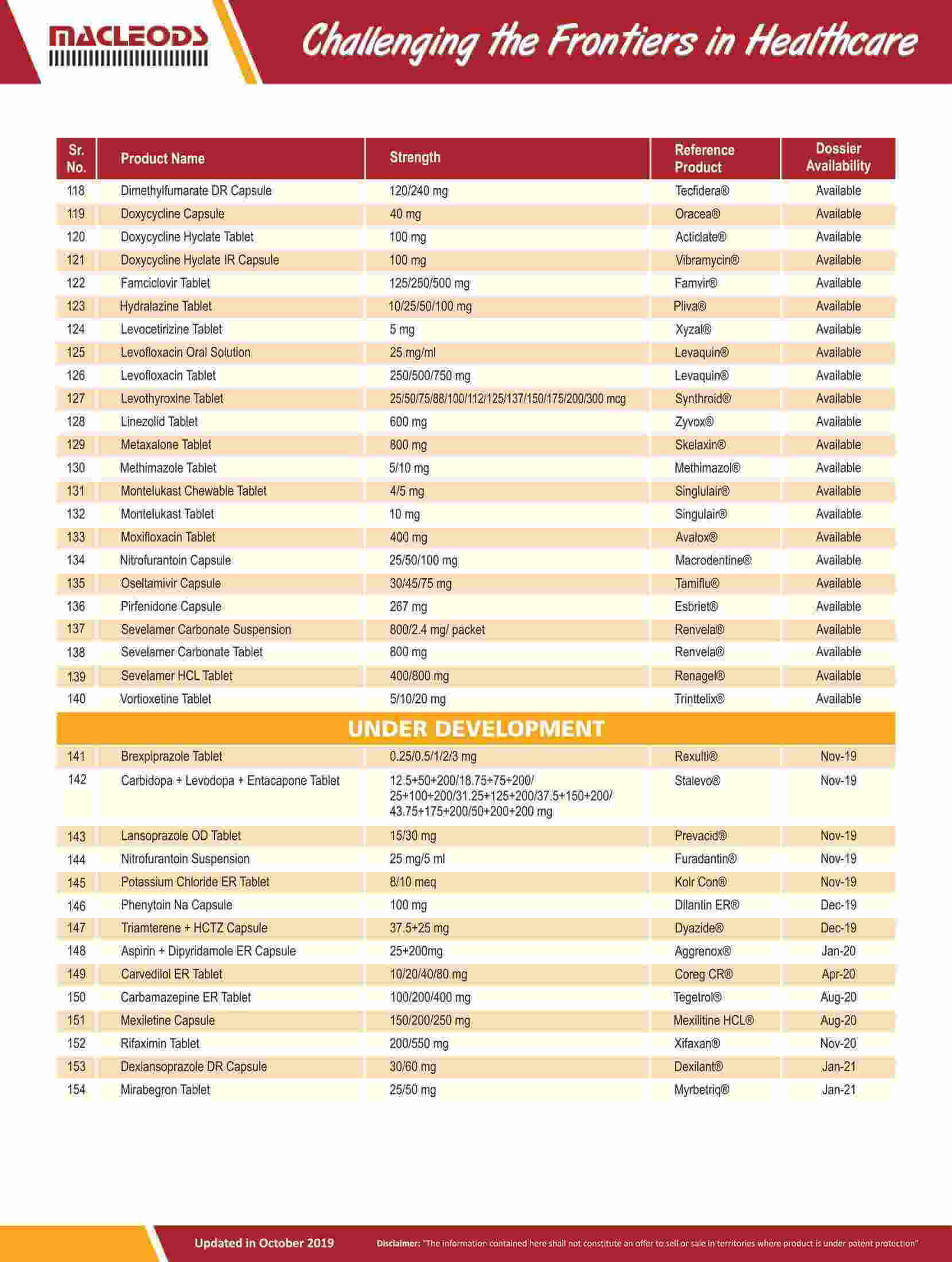
As of August 31, 2023, we had 185 ANDAs filed with the USFDA, of which 100 were approved.
2020 marked the culmination of several block capacity expansions of our facilities for APIs and finished dosages, as we continue to unveil a brand-new manufacturing facility for finished dosages, exclusively for the US market.
Medication Guide
Contact
Report Product Quality, Adverse/ Side Effects
Any Adverse Event/Side Effect/Medical Inquiry And Product Quality Complaint Related to Macleods Product Originating From USA:
USA Direct Line : 1-855-926-3384 (Time 09:00 AM to 05:00 PM CDT From Monday to Friday)
USA Toll Free : 1-888-943-3210 (This is a voice message service. We will respond to voice mail within 24 hours from Monday to Friday, 9 AM to 5 PM CDT)
US Office: +1609 269 5250 (Time: 09:00 AM to 05:00 PM EST From Monday to Friday)
Email : safety@macleodspharma.com